Instructions for Exercise 1:
- To determine the concentration of Solution 1 you should have used the following equation:
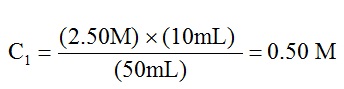
where 2.50 M is the concentration of the stock solution (COld); 10 mL of volume of stock solution were used (VOld) and 50 mL was the volume of the new Solution 1 that was made (VTot).
- To calculate C1,Max and C1,Min you should have used following equations:

where 0.02M is the error in the stock solution (sstock), 0.1 mL is the error of the pipette used (spipette).
- To calculate associated error of C1 you should have used following equation:
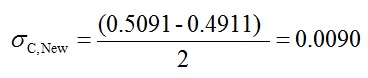
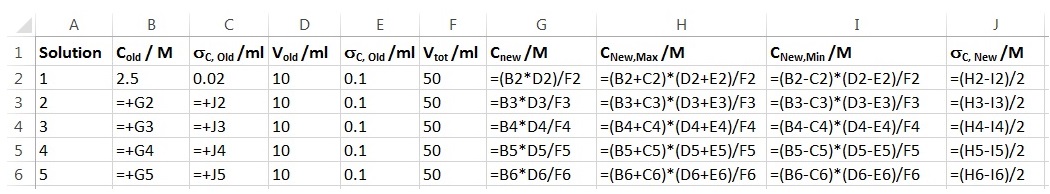
- In Excel in cells G2, H2, I2 and J2 you should have the following formulas:

- For Row 3 we are calculating the concentration and associated error of Solution 2 (which was made by diluting Solution 1). Therefore, the value (COld) in Cell B3 needs to be the concentration of Solution 1. However, rather than typing this number in by hand we can get Excel to copy the exact value across by entering in Cell B3 =+G2 (i.e. Excel will place in Cell B3 the calculated value from Cell G2).
Similarly, the concentration error (sOld) for Solution 1 is entered in Cell C3 using the formula =+J2
Your spreadsheet should look like this:

- The concentration of Solution 2 was calculated using this equation:
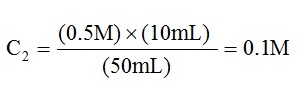
- Equations used in this calculation:
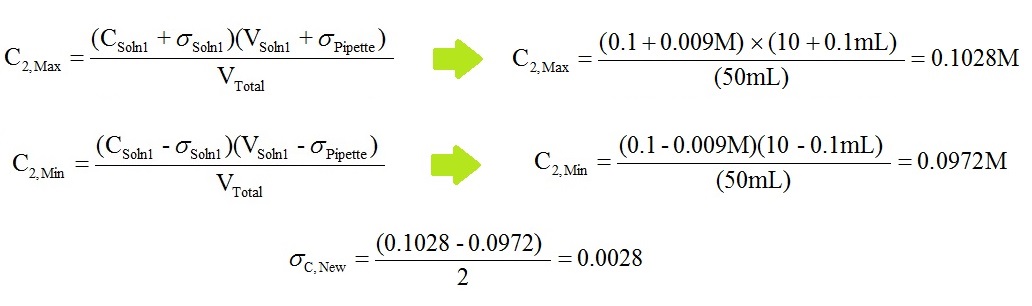
- The numbers in the D, E & F columns will be the same for all the calculations, so highlight D2, E2 & F2 and copy them down to Row 6.
Similarly, the formulae used to calculate the concentrations and errors are the same (just with different numbers), so also copy G2, H2, I2 & J2 down to Row 6.
Finally copy B3 & C3 down to Row 6.
- Your spreadsheet should contain the following formulas:
Now you can go back to Exercise 1.