EXERCISE 2 - Data Analysis - Mean With Associated Error
The experiment of Boyle, Gay-Lussac and their successors showed that the pressure, volume, temperature and molar quantities of perfect gasses are related by the ideal-gas equation:
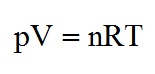
where p = pressure (Pa or Nm-2), V = volume (m3), n = quantity of gas (mol), R = gas constant (8.314 J mol-1K-1), T = temperature (K).
In an experiment, an air-tight, fixed volume vessel was evacuated and then part-filled with gas. The temperature of the vessel was varied, and the pressures recorded as follows:
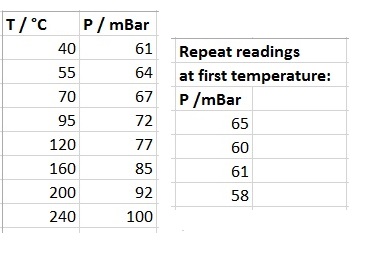
Enter the given data into Excel. Create new columns where temperature and pressure is converted into SI units. Using five pressure readings for the first temperature, calculate the mean pressure along with associated error with 95% Confidence level.
Pressure with associated error is: